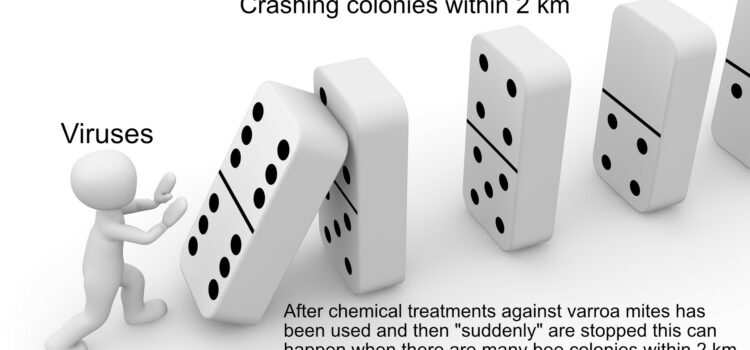
Traits for Varroa resistance We have found in our Varroa resistant project in our local bee club that when you begin a project like this, checking the varroa level is the most important tool, if you plan to treat colonies
Varroa resistance – the two traits